Clinical Development
A total of 84 patients at 25 clinical sites in Germany, Austria, and Russia participated in this randomized controlled phase II efficacy trial in recurrent glioblastoma. The trial compared the efficacy and safety of a combination therapy of asunercept and radiotherapy versus radiotherapy alone.
Patients were eligible for inclusion if they suffered from first or second relapse of glioblastoma and were refractory to standard therapy. Patients randomized into the asunercept arm were treated until further disease progression. Detailed information as well as final results of the trial were published in the peer-reviewed journal "Clinical Cancer Research" (>> publication).
Results
Treatment with asunercept in combination with radiotherapy has shown statistically significant improvements in progression-free survival and quality of life as well as a positive trend in overall survival compared to treatment with radiotherapy alone, demonstrating the potential efficacy of asunercept in the treatment of recurrent glioblastoma. During treatment with asunercept for up to two years, no drug-related serious adverse events were observed, affirming the excellent safety profile and very good tolerability of asunercept.
Both progression-free survival at six months, the primary endpoint of the trial, and median progression-free survival were met with statistical significance. Patients having a newly-identified epigenetic biomarker associated with the CD95 ligand – the target of asunercept – experienced the greatest benefit from treatment with asunercept.
Current Status
Apogenix`partner CANbridge has conducted a multi-center, randomized, double-blind, placebo-controlled phase II study with 119 patients whose objectives were to evaluate the clinical efficacy and safety of asunercept plus TMZ during and after radiation therapy in newly-diagnosed subjects with glioblastoma who have undergone surgical excision. Based on an interim analysis recommendation of the Independent Data Monitoring Committee, CANbridge continued the trial.
Based on the topline data of this trial asunercept did not provide additional benefit in progression-free survival or overall survival over placebo in patients receiving standard of care with temozolomide and radiotherapy.
A phase II efficacy trial with asunercept to treat patients with recurrent glioblastoma has shown statistically significant improvements in progression-free survival and quality of life as well as a positive trend in overall survival.
A scientific publication confirms that the CD95 system – which is inhibited by asunercept – plays an important role in the progression of other solid tumors such as ovarian, colon, prostate, breast, bladder, and kidney cancer.
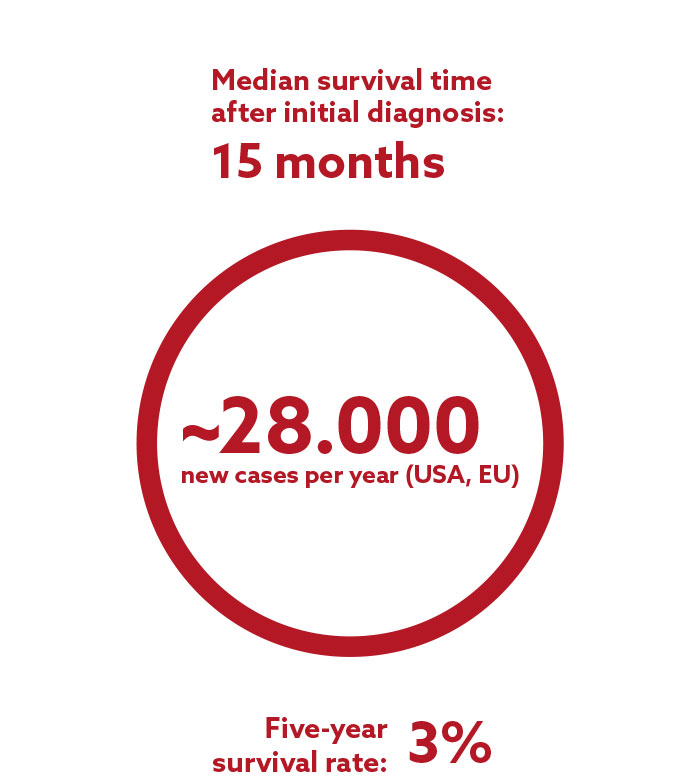
Glioblastoma
Glioblastoma belongs to the group of gliomas and is the most frequent and aggressive type of brain tumor. Glioblastoma cells are highly resistant to radiation and chemotherapy. They spread and infiltrate into the neighboring brain tissue so quickly that a complete surgical removal of the tumor is often impossible. The current standard therapy after initial diagnosis consists of surgery, followed by radiation and chemotherapy. Due to the rapid infiltration of the tumor cells into the surrounding, healthy brain tissue, recurrence is often experienced within a few months of the initial treatment.
There is currently no approved drug for the treatment of recurrent glioblastoma available in the EU, illustrating the tremendous need for new and effective therapies in this indication.