Background/Rationale
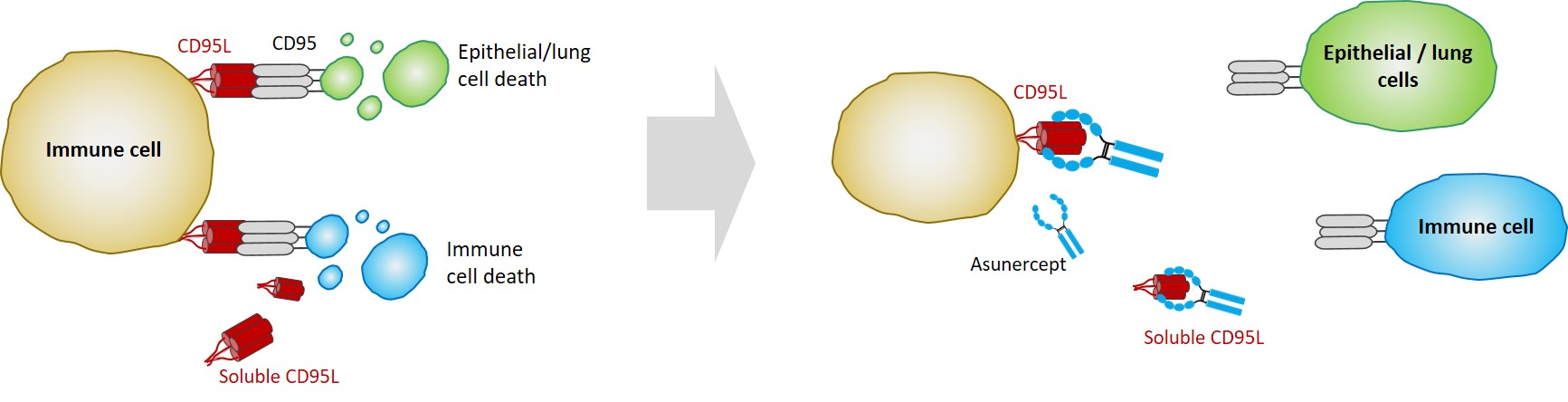
The natural role of the CD95 ligand (CD95L) is to maintain immune homeostasis by induction of apoptotic immune cell death. Dysregulation of this critical CD95L function has been reported in various diseases such as cancer and viral infections (influenza, SARS, MERS, and COVID-19).
In viral infections such as influenza or COVID-19, CD95L causes severe dysregulation of the immune system, resulting in two major pathological problems – reduced lymphocyte counts (lymphopenia) and inflammatory cell death. During disease progression this can lead to pneumonia and acute respiratory distress syndrome (ARDS).
Published data show that CD95L levels are increased in the lung of ARDS patients and cause the inflammatory death of epithelial cells. These data indicate that CD95L plays a major role in the induction of life-threatening lymphopenia and inflammatory cell death in COVID-19 patients.
Asunercept, a CD95-Fc fusion protein, specifically binds to CD95L, efficiently blocking CD95L activity involved in these critical pathogenic mechanisms. Asunercept could therefore represent a unique therapeutic approach for viral infections such as COVID-19, especially as asunercept is not directed against the virus itself, but re-balances the patient's immune response. As a consequence, treatment with asunercept is independent of viral strains.
Clinical Development - COVID-19
ASUNCTIS Phase II
On the basis of this convincing scientific rationale, Apogenix has completed a controlled, randomized phase II study (ASUNCTIS) in hospitalized COVID-19 patients. Patients were randomized into four arms, three with different dose levels of asunercept combined with standard of care compared to standard of care alone. Within just six months, the study protocol was finalized and the first patient was recruited. The study was completed in Q1 2022, with 438 patients being recruited in less than 1.5 years. Final results from the phase II study will be published in a scientific journal.
ASUCOV Phase III
Encouraging results from the interim analysis of the ASUNCTIS phase II study led Apogenix to initiate a pivotal phase III study (ASUCOV), which was supported by a large German federal research grant. ASUCOV was a phase III study in hospitalized COVID-19 patients.
The primary endpoint was an improvement of clinical COVID-19 severity, the secondary endpoints were biomarkers. These included the analysis of calprotectin, which is known as a predictor of the inflammatory stage. Calprotectin has also been described as being correlated with disease outcome in COVID-19.
Given the current development of the COVID-19 pandemic, Apogenix has prematurely terminated the ASUCOV trial. Reasons were that the recent variants of the SARS-CoV-2 virus led to very slow patient recruitment during the first study months and feedback from physicians that the hospitalization duration of eligible patients and the severity of COVID-19 has decreased remarkedly. Hence reaching the recruitment target within an acceptable timeframe was no longer realistic. The premature termination was not decided based on any safety concerns. The risk/benefit ratio of asunercept remains unchanged. Apogenixhas informed all clinical sites as well as the responsible Regulatory Authorities and Ethics Committees and plans to prepare an abbreviated Clinical Study Report in 2024.