Clinical Development
Asunercept underwent a single-arm phaseI/II clinical trial for MDS treatment, conducted at two clinical centers in Germany (Heidelberg and Mannheim). The trial included 20 transfusion-dependent patients with low to intermediate-1 risk MDS, refractory to erythropoiesis-stimulating agents (ESA). Patients received three months of asunercept treatment and were monitored for an additional six months.
The study primarily assessed safety and tolerability, with secondary objectives focused on transfusion frequency and erythropoiesis-related parameters. For comprehensive trial information, including design and participating sites, visit www.clinicaltrials.gov.
During the trial, asunercept treatment was well tolerated, resulting in a 40 percent reduction in transfusion frequency among patients. Measurements of erythropoiesis-related parameters, including progenitor cell numbers and function, demonstrated that asunercept could effectively stimulate erythropoiesis in these MDS patients.
The phase I/II trial has been completed. The final data of the trial were published in March 2018.
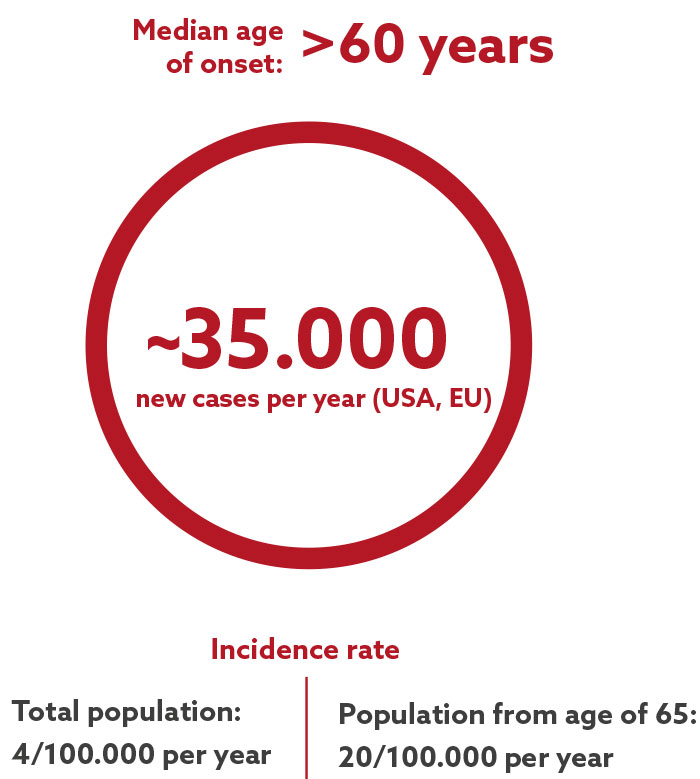
Apogenix’ lead immuno-oncology candidate asunercept is undergoing clinical development to treat solid tumors and malignant hematological diseases, including myelodysplastic syndromes (MDS). MDS is a bone marrow disorder characterized by ineffective hematopoiesis, often leading to severe anemia. This anemia is typically managed with blood transfusions, which can eventually result in iron overload, potentially damaging vital organs.
In MDS, the decrease in thrombocytes responsible for coagulation and leukocytes responsible for immune defense poses a significant health risk. Patients frequently experience sudden bleeding and are vulnerable to life-threatening infections. Additionally, MDS patients are at risk of developing acute myeloid leukemia, a form of blood cancer.
Asunercept's mechanism of action targets the CD95 system, which plays a crucial role in the ineffective hematopoiesis observed in MDS patients. By inhibiting the CD95 system, asunercept directly tackles the root cause of the disorder, offering potential as a curative treatment for MDS.