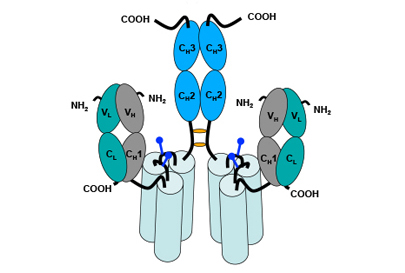
Molecular Structure as a Prerequisite for Appropriate Biological Function
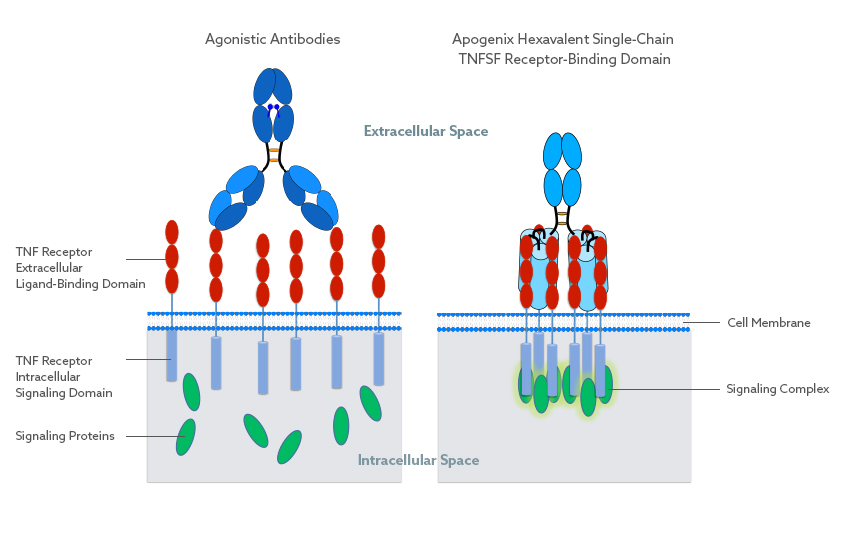
Apogenix has developed a proprietary technology platform for the construction of novel TNF superfamily receptor agonists (HERA-ligands). This single-chain TNFSF (tumor necrosis factor superfamily) technology is superior to other biologics targeting TNFSF pathways, such as agonistic antibodies.
Antibodies can only bind two TNFSF receptors in a spatially undefined manner and require secondary cross-linking via Fcγ receptors.
In contrast, Apogenix’ hexavalent compounds lead to well-defined TNFSF receptor clustering without the need for further cross-linking. This results in a sufficient level of the appropriate signal being transmitted into the target cell, whereas agonistic antibodies transmit these signals at insufficient levels.
Currently, Apogenix utilizes its HERA-ligand platform primarily for the preclinical development of CD40, GITR, and 4-1BB agonists.
Collaborations to advance research and development of these molecules are welcome. The TRAIL program is partnered with AbbVie Inc.
Preclinical efficacy data of Apogenix' HERA-CD27L and HERA-CD40L were published in 2018:
A Single-Chain-Based Hexavalent CD27 Agonist Enhances T Cell Activation and Induces Anti-Tumor Immunity (Frontiers in Oncology)
The Hexavalent CD40 Agonist HERA-CD40L Induces T-Cell-mediated Antitumor Immune Response Through Activation of Antigen-presenting Cells (Journal of Immunotherapy)
Molecular Structure of TNFSF Proteins
TNFSF proteins naturally exist as homo-trimers. Homo-trimers generally consist of three individual, yet identical polypeptide chains, which form one structural unit. As a consequence, TNFSF proteins only show biological activity when their three polypeptide chains are correctly assembled forming one receptor-binding domain (RBD), which in turn binds three receptors.
TNFSF Protein Structure
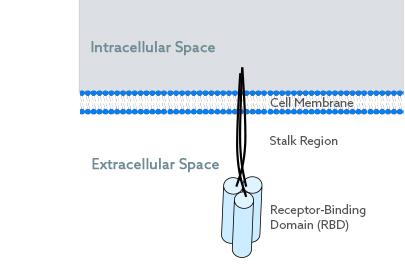
All members of the TNFSF are transmembrane proteins. They have four functional parts in common:
- an intracellular region
- a transmembrane portion
- the stalk region
- the receptor-binding domain (RBD)
The RBD is located extracellularly. It consists of three identical polypeptide chains and comprises three receptor binding sites. Thus, the TNFSF-RBD is considered homo-trimeric and trivalent. The N-terminal end of each of the three polypeptide chains is located inside the cell, whereas the C-terminus is found outside of the cell. Importantly, all three extracellular ends are in close proximity.
Defined Receptor Clustering for Sufficient Signaling
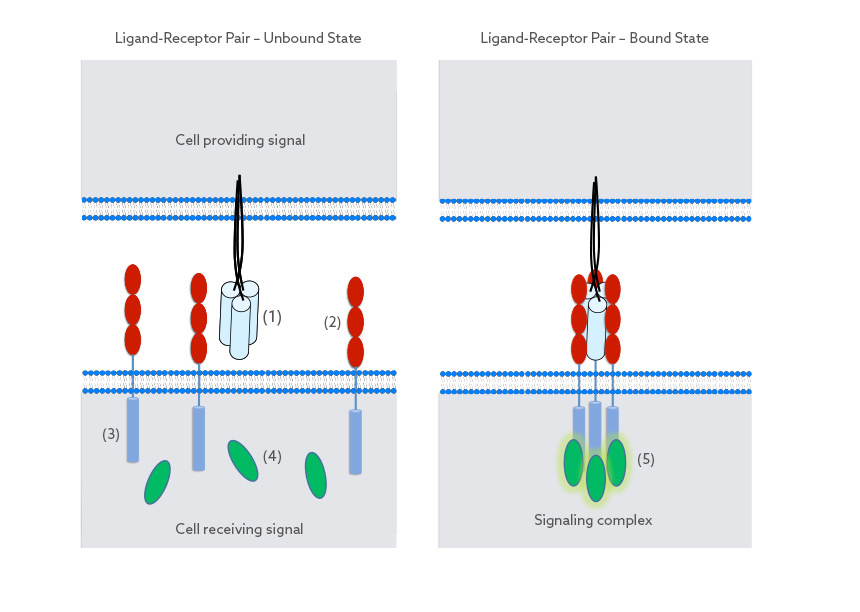
Each trivalent TNFSF-RBD (1) is able to bind three receptors (2). Binding usually occurs in between cells and leads to a defined parallel orientation of the receptors in relation to the RBD. As the distance between binding sites is short, the bound receptors come in close proximity of each other. This lateral clustering of receptors is paramount for the initiation of signaling inside the receiving cell.
Assembly of the clustered receptors outside of the cell in turn leads to close proximity of their intracellular portions (3). This intracellular clustering allows signaling proteins (4) inside the cell to be recruited (5), thereby initiating a response from the cell. As a consequence, the strength as well as the quality of the initiated signal is driven by the well-defined receptor clustering outside of the cell.
From Trimeric to Hexavalent – the Single-Chain TNFSF-RBD
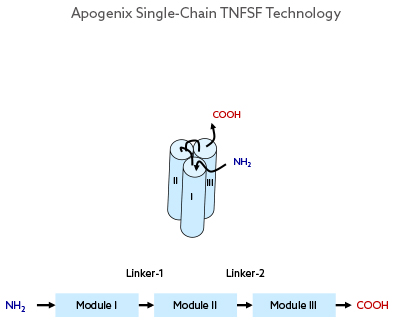
The Apogenix single-chain TNFSF (scTNFSF) technology is designed in a way that resembles the natural TNFSF receptor-binding domain (TNFSF-RBD). This is achieved by depleting the intracellular portion and the stalk region from the full-length TNFSF polypeptide. The remaining RBD-forming subsequence of the TNFSF protein is then used to genetically build a single polypeptide with three repetitive RBD-forming sequence modules connected by two short peptide linkers. This artificial TNFSF-RBD module is monomeric (one polypeptide chain), but trivalent when folded as a protein.
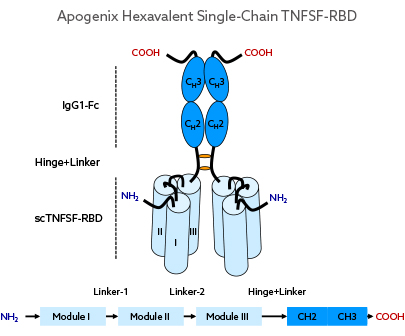
By fusing the Fc-domain of human IgG1 to the C-terminal end of the scTNFSF-RBD, a homo-dimeric but hexavalent drug unit is obtained.
Enhanced TNFSF Receptor Clustering Through Single-Chain TNFSF-RBD
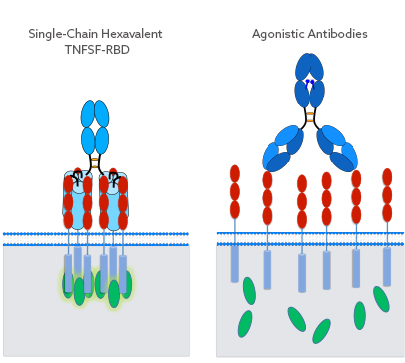
The scTNFSF-RBD-Fc molecular layout mimics the characteristic and well-defined receptor clustering of TNFSF proteins, resulting in a sufficient level of the appropriate signal being transmitted into the target cell. This is in contrast to other biologics targeting TNFSF pathways, such as agonistic antibodies, as these can only bind two receptors per drug molecule in a spatially undefined manner.
Multiple Protein Formats for Multiple Purposes
The Apogenix protein engineering concept allows for the creation of a plethora of anti-cancer biologics, including trivalent and hexavalent protein formats with different pharmacodynamic and pharmacokinetic properties.
Monomeric Trivalent scTNFSF-RBD
This is the basic building block which enables symmetrical positioning of additional protein domains on either end of the polypeptide chain. This format can be produced as a single biologically active compound with a short serum half-life (hours) due to its overall low molecular weight (approx. 60 kD). There are co-stimulatory TNFRSF targets (e.g., CD40) where trivalent agonists can already achieve target-specific signaling, albeit less effectively than the hexavalent formats. The combination of a short in vivo half-life with reduced agonism can be an advantage, depending on the target and indication being addressed.
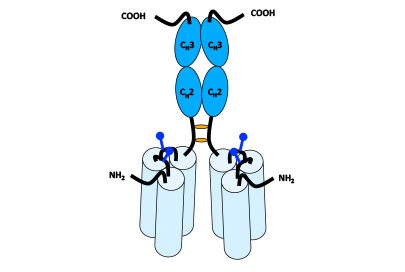
Dimeric Hexavalent scTNFSF-RBD-Fc
Fusing the Fc domain of human IgG1 to the C-terminal end of the scTNFSF-RBD results in a homo-dimeric, but hexavalent drug unit with an overall antibody-like shape and volume. Different muteins of the human IgG1 domain can be employed to create drug candidates with specific FcγR or FcRn binding profiles.
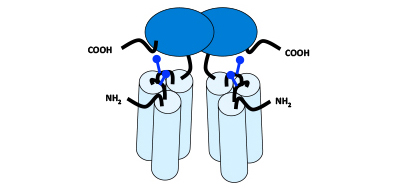
Dimeric Hexavalent scTNFSF-RBD-DIM
By fusing a proprietary dimerization scaffold (DIM), which lacks any Fc functionality, to the C-terminal end of the scTNFSF-RBD, a unique format with an intermediate biological half-life (hours instead of days) and Fc-independent distribution profile can be generated.
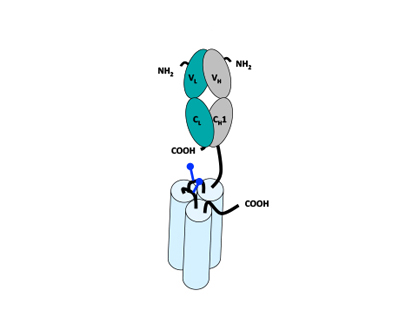
Monomeric Trivalent Fab-scTNFSF-RBD
Tumor-targeting molecules that recognize specific tumor antigens can also be generated by fusing Fab antibody fragments to the N-terminal end of the scTNFSF-RBD. Since this molecule lacks Fc functionality, faster clearance of the drug can be achieved. The resulting molecule is monovalent for the tumor-specific antigen and trivalent for the TNFRSF member being targeted. Being trivalent, the overall agonistic activity of the compound is reduced in circulation, but enhanced upon its immobilization on tumor cells. Binding of the antibody fragment leads to a local enrichment (or clustering) of the trivalent agonist on the tumor cell surface. These local clusters of the scTNFSF-RBD on tumor cells are therefore multivalent for their co-stimulatory receptor target. Agonistic activity is enhanced upon contact between the tumor and immune cells, with the immune cell receiving the appropriate signal by the scTNFSF-RBD moiety of the drug.
Dimeric Hexavalent Fab-scTNFSF-RBD-Fc
Where necessary, the scTNFSF-RBD can be combined with a full-length antibody, resulting in a bispecific drug that is bivalent for the antigen recognized by the Fab domain and hexavalent for the TNFRSF member being targeted.